
Enhancement of the anti-tumour immune response with moderate hyperthermia
The use of the hyperthermia as a complement to the cancer immunotherapy is supported by a growing body of research data. Both preclinical and clinical data results have demonstrated enhanced anti-tumour immune responses with the addition of moderate hyperthermia. The molecular mechanisms responsible for the enhanced immune reactivation observed in the presence of hyperthermia include the generation of Hsps (a family of proteins that are produced by cells in response to exposure to stressful conditions such as heat shock), activation of antigen-presenting cells and changes in lymphocyte trafficking.
Elevated body temperatures have been recognised as beneficial components of the immune defence response against pathogenic stimuli since ancient times.and the notion of treating human cancers with heat dates back to the writings of Hippocrates. However, heat as a treatment modality for cancer has only begun to be rigorously evaluated in the last few decades.. There is renewed interest in the application of heat to improve the efficiency of standard cancer therapies, such as chemotherapy and radiotherapy.. The combination of immunotherapy with hyperthermia for the treatment of cancer, however, is a particularly intriguing notion, as it the significant clinical effects of hyperthermia have been attributed to the immune system.. The accepted view of the cancer-host immune interface is that tumours possess unique antigens that can be recognised by the immune system. After antigen uptake at tumour sites, APCs or antigen-presenting cells (also called dendritic cells) have the ability to create a potent response by entering the lymphoid compartments and programming lymphocytes. After generation and expansion to very large numbers, cytotoxic lymphocytes can then travel to the tumour site to kill cancer cells.
To understand how temperature can influence the immune system, it is necessary to define the concept of hyperthermia. As the father of clinical thermometry, Wunderlich is credited with defining normal body temperatures at 37°C and describes a dynamic range of normal body temperatures with diurnal variations. Fever induces elevation of the physiological set point of body temperature, raising core body temperatures through specific thermoregulators. Hyperthermia differs fundamentally from fever in that it raises core body temperature without changing the physiological set point.. Normally, hyperthermia is induced by increasing the heat load and/or inactivating heat dissipation.

Early studies of hyperthermia focused on the cytotoxic effects of high temperatures and direct tumour cell death.. Although significant cell death could be achieved by heating cells or tissues to temperatures > 42°C for 1 or more hours, the application, measurement and consistency of this temperature range within the cancer clinical trial setting proved problematic. Unless thermal ablation of tumour tissue is applied within a localised area, hyperthermia in the cytotoxic range could not be reliably achieved in tumours of heterogeneous size and tissue type. Therefore, hyperthermia at mild temperature (i.e. within the fever range, 39-41°C) and moderate hyperthermia (41°C) have emerged as focal points for ongoing clinical investigations.as they are easily achievable and tolerated. We focus on Hsps and APCs and improved immunotherapy strategies.
Cellular functions of Hsps
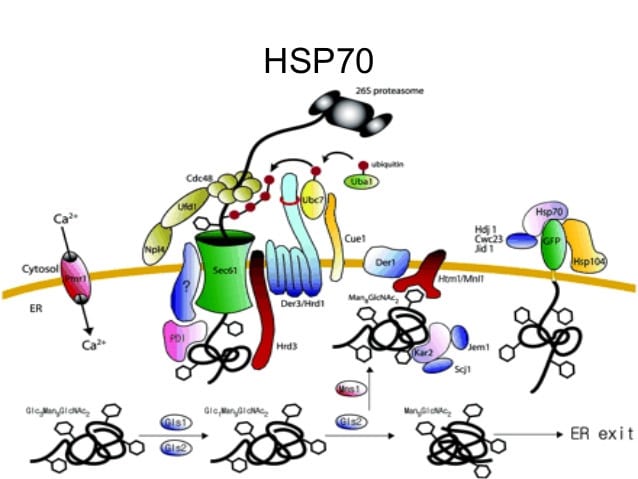
Hsps are a family of stress-induced proteins with several critical cellular functions, and are typically designated by their molecular weight. Hsps were discovered in 1962 as a result of the accidental application of heat stress to Drosophila preparations. In the last 30 years, Hsps have been characterised in a variety of cells, in species ranging from prokaryotes to humans, and are highly conserved. Hsps attenuate the effects of cellular stressors. They are now recognised as central mediators of a variety of cellular functions under physiological conditions, as they are the main regulators of cellular protein activity. During homeostasis, Hsps ensure proper post-translational protein folding, and are able to refold denatured proteins, or mark irreversibly damaged proteins for destruction. Not surprisingly, given their crucial cellular activities, Hsps represent one of the most abundant protein types in unstressed cells, accounting for 1-2% of all cytosolic proteins. Defining the function of Hsps in cancer cells has become a burgeoning area of research.as described below.
Hsps in cancer
The activities of Hsps in transformed tumour cells are complicated and diverse. The Hsps are present in abundance in various types of tumours and may confer several survival benefits to cancer cells.. There is evidence that a specific Hsp, Hsp70, directly inhibits apoptosis pathways in cancer cells, as has been shown in human pancreatic cancer, prostate and gastric cancer cells.. Hsps have also been implicated in mediating resistance to potentially cytotoxic hyperthermia in a process called thermotolerance. More specifically, the synthesis and accumulation of Hsps in tumour cells exposed to hyperthermia may provide protection against other heat-associated cytotoxic events, as Hsps can rescue or restore vital cellular proteins. Thermotolerance has the capacity to generate a population of tumour cells that are refractory to subsequent hyperthermic changes. Moreover, there is evidence that Hsps support the malignant phenotype of cancer cells, not only affecting cell survival, but also participating in angiogenesis, invasion, metastasis and immortalisation mechanisms. Contrary to the many benefits conferred to tumour cells expressing high levels of Hsps, the dependence of tumour cells on Hsps for several critical functions represents an attractive and potential therapeutic target; a virtual Achilles' heel.
Hsps and the immune system
The immune system has evolved to take advantage of the ability of Hsps to act as "danger signals", allowing the generation of an amplified immune response.. Hsp released from stressed or dying cells activate dendritic cells (DCs), transforming them into mature APCs. Endocytosis of Hps by DCs increases cell surface expression of class II MHC molecules, in addition to several co-stimulatory molecules, which enhances immune recognition of antigens. Mature dentritic cells (DCs) can programme lymphocyte effector cells in an antigen-restricted manner, thus limiting collateral damage to normal healthy tissues, which do not express the target antigen. The ability of Hsps to accompany proteins prior to endocytosis and processing by DCs can potentially broaden the repertoire of epitopes presented and thus the spectrum of the immune response.
At Biosalud Day hospital we have incorporated hyperthermia into our specific techniques for the complementary treatment of cancer, forming part of a protocol developed by our R+D+i Department, increasing up to 10 times the effectiveness of dendritic cells in terms of their capacity to destroy cancer cells, increasing angiogenesis and therefore oxygenation of the tumour area (the opposite of what cancer cells need) and starting up the apoptosis mechanisms of the cancer cells.
All this means that Biosalud Hospital de Día can apply the most appropriate protocol in a personalised way. The use of a treatment that complements the conventional treatment prescribed by the oncologist in the most effective way, while greatly reducing the side effects of the treatment.
In cancer, we try to add complementary techniques that offer us the best chance of success, given the complexity of each case.

