Blockage of calcium flux to mitochondria
A new study shows that blocking the transfer of calcium ions (Ca2+) to the mitochondria is toxic to cancer cells. and affects tumour growth in mice, without affecting normal cells.
All cells use calcium ions as signalling agents to regulate metabolism and other cellular functions. By blocking the flow of calcium into mitochondria, which are the main producers of energy-rich ATP molecules in cells (molecules used by all living organisms to provide energy in chemical reactions), an energy 'crisis' was created from which normal cells were able to recover, but the cells of cancer could not, according to the study.
The new findings suggest that mitochondrial calcium addiction is a novel feature of cancer cells that could be exploited to develop new, targeted cancer therapies, study senior author Kevin J. Foskett, Ph.D., of the University of Pennsylvania, said in a news release.
The results of the study were published in Cell Reports 3 March.
A cellular energy crisis
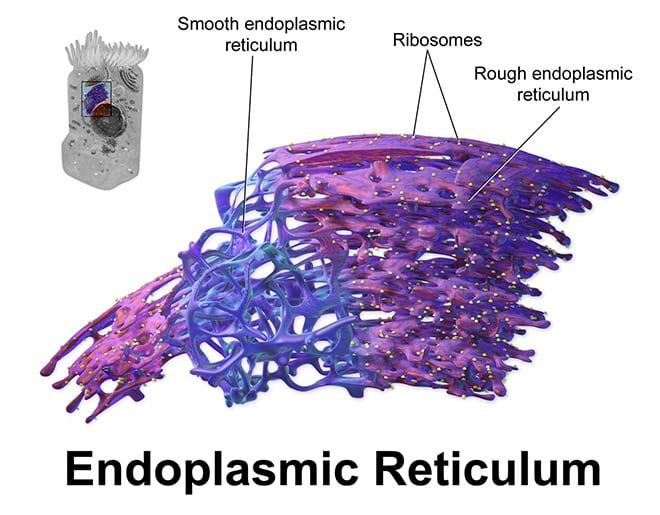
Mitochondria are compartments (organelles) within cells that break down nutrients to produce energy in the form of ATP, which cells use for biochemical power reactions. Previous lab work by Dr. Foskett showed that calcium ions are constantly transported into the mitochondria from another cellular compartment, the endoplasmic reticulum, or ER. This course of calcium transfer between the ER and the mitochondria helps maintain ATP production through a metabolic process called oxidative phosphorylation.
Specialised receptor proteins called InsP3Rs form channels that cross the ER membrane and allow calcium to flow out in a controlled manner. Calcium ions released from the ER then move into adjacent mitochondria.
Working with cell lines, the research teamco-directed by César Cárdenas, Ph.D., Universidad de Chile, used a compound found in sea sponges, called XEB, which inhibits InsP3Rs, preventing the flow of calcium from the ER to the mitochondria.. The effects of XEB were examined in human cancer cell lines derived from breast and prostate cancers, in human fibroblast cells that had been transformed into cancer cells and in comparable normal cells.
When the flow of calcium to the mitochondria was blocked by XEB, both cancer cells and normal cells experienced a "bioenergetic crisis" characterised by reduced ATP production and other slower cellular metabolic signals. Confirming Dr Foskett's previous lab work, normal cells survived this energy crisis by activating a mechanism known as autophagy. In autophagy, or "self-feeding," cells recycle cell components to maintain energy levels under conditions of starvation or stress. Cancer cells also activated autophagy when calcium influx was blocked, but the self-feeding response was not enough to prevent cancer cell death.
Other experiments showed that blocking the calcium transfer caused normal cells to stop dividing, which is an expected response when energy is scarce. In contrast, cancer cells treated in the same way continued to divide, but then underwent cell death during the last phase of cell division.
Using a mouse model of melanoma, the researchers also found that blocking InsP3R activity through a single injection of XEB reduces tumour size by nearly 60 percent in one day compared to untreated tumours. In another series of experiments in mice, treatment with XEB every other day for a week reduces melanoma tumour size by 70 per cent compared to untreated tumours in the same animal.
An unexpected therapeutic target
"Our studies suggest the existence of new and completely unexpected targets by which drugs could be developed to kill cancer cells specifically targeting calcium release from the ER and calcium uptake by mitochondria," Dr. Foskett said.
"This work and previous work by other groups provides compelling evidence that calcium signalling is altered in cancer cells"said Gregory Monteith, Ph.D., of the University of Queensland, Australia, who was not involved in the study.
"In the specific case of calcium transfer from the ER to the mitochondria, we now need to develop a better understanding that proteins in this pathway could be targeted pharmacologically and ensure that, when they are modulated, this will not have substantial effects on other cell types that could result in prohibitive side effects," said Dr Monteith.
Dr. Foskett and his team are working to design high-throughput 'cell line-based' assays (lab tests) to test potential drugs that could affect one or more of these new targets. "We also have plans to partner with cancer biologists to develop some better animal models to test our hypothesis and move forward in different types of cancer, to see if what we've seen in cell lines and in one type of tumour holds up in more robust models.
"Understanding how communication patterns between organelles are maintained or altered in cancer cells is an emerging area in the field of cancer biology," said Michael Espey, Ph.D., of the Cancer Cell Biology Branch in NCI's Division of Cancer Biology. "The finding that cancer cells become overtly dependent on calcium ion flux from ER-to-mitochondria opens up new avenues for designing treatments that could exploit this vulnerability."

