Oxygen suppresses anti-cancer activity of T-cells
Researchers have identified a mechanism in mice whereby anti-cancer immune responses are inhibited inside the lungsa common site of metastasis for many cancers. This mechanism involves oxygen inhibition of the anti-cancer activity of T cells. Inhibition of the oxygen-sensing capacity of immune cells, either genetically or pharmacologically, prevented lung metastasis. This research was carried out by Nicholas Restifo, M.D., Cancer Research Center, University of California, Berkeley, USA.The findings were published in the journal Cell, National Cancer Institute (NCI) and others at the NCI, as well as colleagues at the National Institute of Allergy and Infectious Diseases, both part of the National Institutes of Health. The findings were published on 25 August 2016, in the journal Cell.
Metastasis is the cause of most cancer deaths. It has long been hypothesised that the process of cancer metastasis requires cooperation between the spread of cancer cells and the cellular environment into which they spread. A key component of that environment is the local immune system, which can act to fight invading cancer cells.
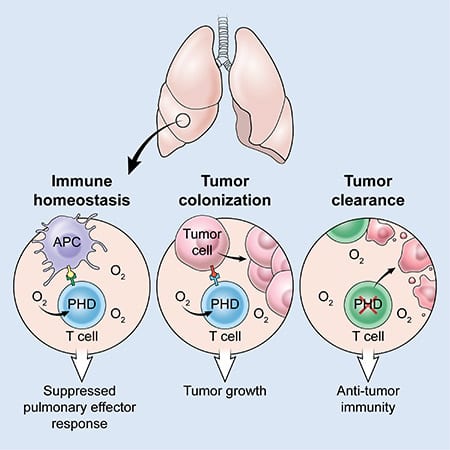
The researchers discovered that T cells, a type of immune cell, contain a group of oxygen-sensing proteins that act to limit inflammation inside the lungs. This new research showshowever, that oxygen also suppresses the anti-cancer activity of T-cells, allowing cancer cells that have spread to the lungs to escape immune attack and establish metastatic colonies.
"Since the lung is one of the most frequent sites to which cancers spread, we hypothesised that there may be unique immune processes that aid tumour cells in their ability to establish themselves in the lung. Because oxygen is a pervasive environmental factor in the lung, we wanted to examine what role oxygen might play in the regulation of immunity in the lung. "said David Clever, M.D., who trained in Restifo's lab and has now returned to the Ohio State University College of Medicine.
The research team discovered that oxygen-sensing proteins, called prolyl hydroxylase domain (PHD) proteins, act within T cells to prevent excessively strong immune responses to harmless particles that frequently enter the lung. This protective mechanism also allows circulating cancer cells to gain a foothold in the lung. Specifically, the researchers found that PHD proteins promote the development of regulatory T cells, a type of T cell that suppresses the activity of other parts of the immune system. They also found that PHD proteins limit the development of inflammatory T cells and restrict their ability to produce molecules involved in cancer killing.
To test whether PHD proteins promote tumour cells to grow in the lung, the researchers used a knock-out (KO) mouse strain that lacks PHD proteins in its T cells. These PHD knock-out mice, as well as unaltered normal mice, were injected with melanoma cells. Surprisingly, while the normal mice showed large numbers of melanoma cancer cells in their lungs, mice whose T cells lacked PHD proteins showed almost no evidence of melanoma in the lungs.
Given their finding that PHD proteins suppress the inflammatory immune response in the lung, the researchers wondered whether their inhibition might improve the efficacy of adoptive cell transfer, a type of immunotherapy which harnesses the ability of T cells themselves to recognise and attack cancer. In adoptive cell transfer, T cells are extracted from a patient's tumour tissue, expanded to large numbers in the laboratory, and then administered intravenously to the patient along with a T-cell growth factor, in the hope that these cells will return to the cancer sites and kill it.
For these experiments, the research team expanded anti-tumour T cells in the presence of a drug called dimethyloxaloylglycine (DMOG), which blocks the activity of PHD proteins. In the laboratory, drug treatment improved the cancer-killing properties of T cells and when given to mice with established metastatic cancer, drug-treated T cells were much better able to kill cancer than untreated T cells. Treatment with DMOG has also been found to improve the cancer-killing properties of human T cells in other studies. The application of these findings to clinical trials of human adoptive cell transfer immunotherapy is being investigated by Restifo's group.
"Adoptive cell transfer immunotherapy provides a unique opportunity for manipulation of a patient's own T cells outside the body," said Restifo. "Although our finding has been made in mice, we are eager to test whether disrupting the oxygen-sensing machinery in T cells - with drugs, genetics or environmental oxygen regulation - will increase the efficacy of immune therapies. T-cell-mediated cancer treatment in humans".
In the video, Nick Restifo, PhD, a senior researcher at the US National Cancer Centre, discusses his recently published study finding that oxygen, a molecule necessary for life, paradoxically helps cancer metastasise in the lung by damaging cancer-killing immune cells.
Source: NIH

